Contact
About Eric Tucker
https://medicine.hsc.wvu.edu/neuroscience/faculty-labs/eric-s-tucker-phd/
Positions
Assistant Professor
- Organization:
- West Virginia University School of Medicine
- Department:
- Rockefeller Neuroscience Institute (SOM)
- Classification:
- Faculty
Education
- PhD, University of Arizona
Publications
[2020]
- Myers AK, Cunningham JG, Smith SE, Snow JP, Smoot CA, Tucker ES. (2019). JNK signaling is required for proper tangential migration and laminar allocation of cortical interneurons. Development 147-2.
- This article has associated cover art.
- Nolan RL, Brandmeir N, Tucker ES, Magruder JL, Lee MR, Chen G, Lewis JW. (2019) Functional and resting-state characterizations of a periventricular heterotopic nodule associated with epileptogenic activity. Neurosurg Focus 1:48-2.
[2019]
- Moye AR, Bedoni N, Cunningham JG, Sanzhaeva U, Tucker ES, Mathers P, Peter VG, Quinodoz M, Paris LP, Coutinho-Santos L, Camacho P, Purcell MG, Winkelmann AC, Foster JA, Pugacheva EN, Rivolta C, Ramamurthy V. (2019). Mutations in ARL2BP, a protein required for ciliary microtubule structure, cause syndromic male infertility in humans and mice. PLoS Genet 15-8.
[2015]
- Lucke-Wold BP, Nguyen L, Turner RC, Logsdon AF, Chen YW, Smith KE, Huber JD, Matsumoto R, Rosen CL, Tucker ES, Richter E. (2015). Traumatic brain injury and epilepsy: Underlying mechanisms leading to seizure. Seizure 33: 13-23.
- Meechan DW, Maynard TM, Tucker ES, Fernandez A, Karpinski BA, Rothblat LA, LaMantia AS. (2015) Modeling a model: Mouse genetics, 22q11.2 Deletion Syndrome, and disorders of cortical circuit development. Prog Neurobiol 130: 1-28.
[2014]
- Myers AK, Meechan DW, Adney DR, Tucker ES. Cortical interneurons require Jnk1 to enter and navigate the developing cerebral cortex. J Neurosci (2014 June) 34(23:7787-7801. doi: 10.1523/JNEUROSCI.4695-13.2014.
- This article has associated cover art and cover video.
[2012]
- Meechan DW, Tucker ES, Maynard TM, LaMantia AS. Cxcr4 regulation of interneuron migration is disrupted in 22q11.2 deletion syndrome. Proc Natl Acad Sci USA (2012 Nov 6) 109(45): 18601-6. doi: 10.1073/pnas.1211507109.
[2011]
- Meechan DW, Maynard TM, Tucker ES, LaMantia AS. Three phases of DiGeorge/22q11 deletion syndrome pathogenesis during brain development: patterning, proliferation, and mitochondrial functions of 22q11 genes. Int J Dev Neurosci (2011 May) 29(3): 283-94. doi: 10.1016/j.idevneu.2010.08.005.
[2010]
- Tucker ES, Lehtinen MK, Maynard TM, Zirlinger M, Dulac C, Rawson N, Pevny L, LaMantia AS. (2010) Proliferative and transcriptional identity of distinct classes of neural precursors in the mammalian olfactory epithelium. Development 137:2471-2481.
- Rawson NE, Lischka FW, Yee KK, Peters AZ, Tucker ES, Meechan DW, Lehtinen MK, Zirlinger M, Maynard TM, Burd GD, DuLac C, Pevny L, LaMantia AS. (2010) Specific mesenchymal/epithelial induction of olfactory receptor, vomeronasal, and gonadotropinreleasing hormone (GnRH) neurons. Developmental Dynamics 239(6):1723-38.
[2009]
- Meechan DW, Tucker ES, Maynard TM, LaMantia AS. (2009) Diminished dosage of 22q11 genes disrupts neurogenesis and cortical development in a mouse model of 22q11 Deletion/DiGeorge Syndrome. Proceedings of the National Academy of Sciences 106(38):16434-16439.
[2008]
- Tucker ES, Segall S, Gopalakrishna D, Wu Y, Vernon M, Polleux F, LaMantia AS. (2008) Molecular specification and patterning of progenitor cells in the lateral and medial ganglionic eminences. The Journal of Neuroscience 28(38):9504-9518.
[2006]
- Tucker ES, Polleux F, LaMantia AS. (2006) Position and time specify the migration of a pioneering population of olfactory bulb interneurons. Developmental Biology 297(2):387- 401.
- Councill JH, Tucker ES, Haskell GT, Maynard TM, Meechan DW, Hamer RM, Lieberman JA, LaMantia AS. (2006) Limited influence of olanzapine on adult forebrain neural precursors in vitro. Neuroscience 140 (1):111-22.
Additional Info
News & Noteworthy
06/04/2014: Tucker lab publishes cover article in The Journal of Neuroscience

Associated press releases:
- http://westvirginia.mdnews.com/press-releases/wvu-lab-finds-possible-link-to-developmental-brain-disorders.aspx
- http://www.wdtv.com/wdtv.cfm?func=view§ion=Fox-10&item=WVU-Researchers-Find-Possible-Link-to-Brain-Disorders-16403
- http://www.sciencedaily.com/releases/2014/06/140611151113.htm
- http://www.autismdailynewscast.com/autism-research-june-14-2014-week-in-review/12445/robertahill/
Open Positions
**********************************
Biology Technician (NEUROA 15-0070)
The West Virginia University Research Corporation (WVURC) seeks to hire a Biology Technician in the Department of Neurobiology & Anatomy at West Virginia University. Responsibilities will include maintaining a transgenic mouse colony, ordering supplies and reagents, preparing media and solutions, and performing routine lab experiments under the PI's direction.
A bachelor's degree in biology or related field, and 0-2 years of relevant research experience in a biomedical research lab setting are required. An equivalent combination of education and experience will be considered. Hands-on experience in mouse handling techniques, tissue culture, molecular biology, or immunocytochemistry is preferred.
Competitive salary and benefits package offered. For a complete job description and to apply for this position, please visit http://hr.research.wvu.edu and click on the "WVURC Employment Opportunities" link. AA/EOE/Minorities/Females/Vet/Disability/E-verify compliant employer
************************************
We are currently seeking outstanding graduate and undergraduate students to join our lab! Interested individuals should contact Eric Tucker for details.
Research Program
Neuroscience
Research Interests
My laboratory takes cellular, molecular and genetic approaches to study mammalian forebrain development. In particular, we focus our attention on GABAergic interneurons of the cerebral cortex, which are key cellular targets in the pathogenesis of multiple neurodevelopmental disorders including schizophrenia and autism. Our goal is to identify molecular mechanisms that underlie the generation, migration, and differentiation of cortical interneurons, and provide insight into how disruptions in cortical development may result in neurological and psychiatric illnesses.
Techniques include:
- Mouse genetics/embryology
- Electroporation
- Organotypic tissue culture
- Primary neuronal cell culture
- Immunocytochemistry
- Molecular biology
- Biochemistry
- Confocal microscopy
- Live-cell imaging
Time-lapse confocal imaging reveals dynamic behavior of cortical interneurons as they migrate into the developing cerebral cortex.
Role of JNK signaling in cortical development and function
Our laboratory recently discovered a prominent role for c-Jun N-terminal kinase (JNK) signaling in directing the early migration of cortical interneurons into and within the developing cerebral cortex. We identified Jnk1 as a positive regulator of interneuron migration in vivo, where it acts cell autonomously to promote the entry of interneurons into the cerebral cortex and the formation of coherent migratory streams. Time-lapse imaging revealed that cortical interneurons remain motile but lose their navigational capacity following pharmacologic JNK inhibition, suggesting the JNK pathway acts downstream of cell surface guidance receptors to orient the migration of cortical interneurons.
A major focus of the lab is currently aimed at identifying upstream activators and downstream effectors of JNK signaling in migratory cortical interneurons. By identifying JNK activators and effectors we will elucidate cellular and molecular pathways critical for the guided migration of cortical interneurons. We are also studying the mature brain of JNK-deficient animals in order to determine the ultimate consequences for alterations in cortical interneuron migration following JNK inhibition during development. By characterizing the anatomical, physiological, and behavioral consequences of JNK deficiency, we will determine the extent to which disrupted JNK signaling compromises cortical function. Together, these studies will add key insight into genetic mechanisms underlying the formation and function of cortical circuitry in normal and pathologic conditions.
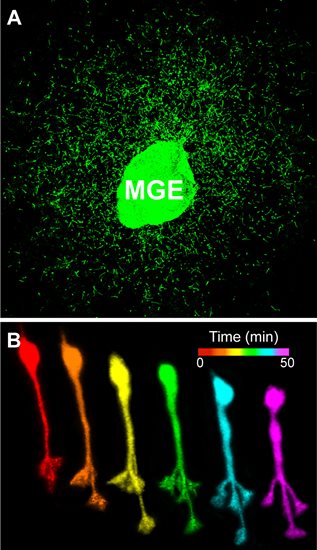
Visualizing migratory properties of cortical interneurons. A. Cortical interneurons migrate from an explant of medial ganglionic eminence (MGE) tissue in vitro. B. Time-lapse sequence of an individual cortical interneuron undergoing nucleokinesis and leading process branching.